Research overview:
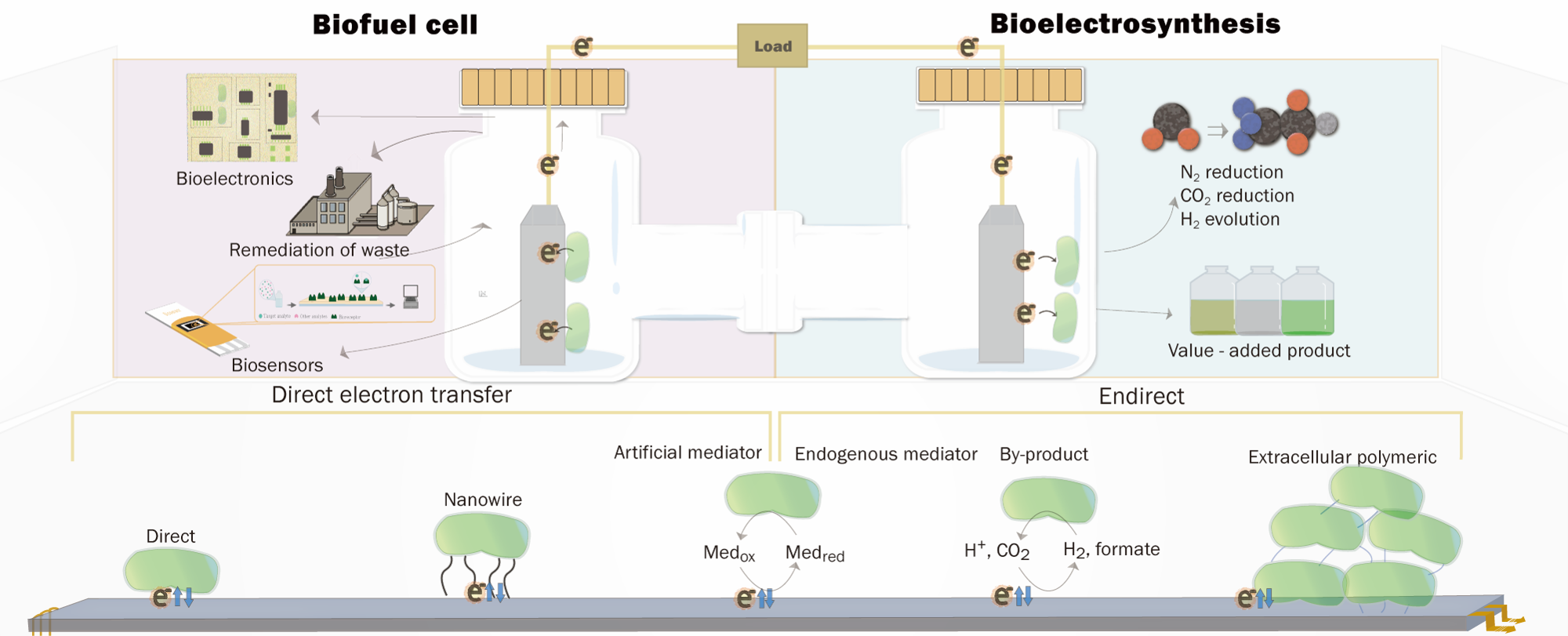
Research in the Lee Group seeks to couple metalloenzymes that catalyze important reductive reactions to electrodes, in order to study their electron transfer/catalytic mechanisms and ultimately aid the development of new biotechnologies (and bio-inspired technologies).
Bioelectrosynthesis:
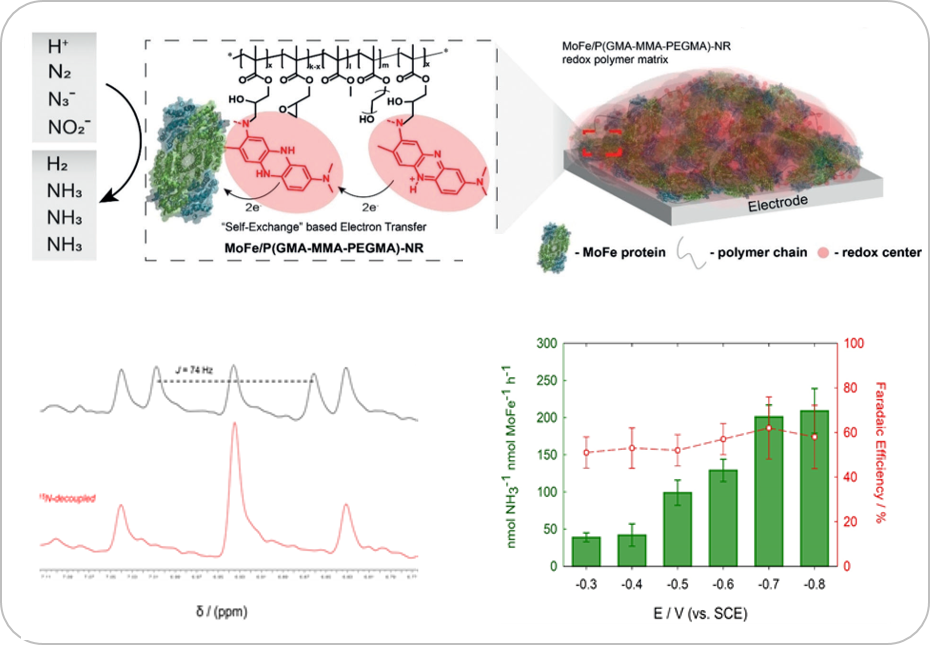
Our multidisciplinary research combines skills ranging from recombinant protein production to enzymatic electrochemistry to study enzymes that catalyze reactions such as (i) dinitrogen (N2) reduction to ammonia (NH3), (ii) carbon dioxide (CO2) reduction, and (iii) large metalloenzyme complexes that employ non-trivial electron transfer mechanisms.
In enzymatic electrochemistry, electrodes can be electronically coupled to metalloenzymes in many different ways although the desired outcome remains the same: the electrode supplies the reducing equivalents to the reductive metalloenzyme for subsequent catalysis (or, electrocatalysis) where the corresponding current is proportional to the rate of substrate reduction by the enzyme
In enzymatic electrochemistry, electrodes can be electronically coupled to metalloenzymes in many different ways although the desired outcome remains the same: the electrode supplies the reducing equivalents to the reductive metalloenzyme for subsequent catalysis (or, electrocatalysis) where the corresponding current is proportional to the rate of substrate reduction by the enzyme
[Selected publications in this area]

Biosensor:
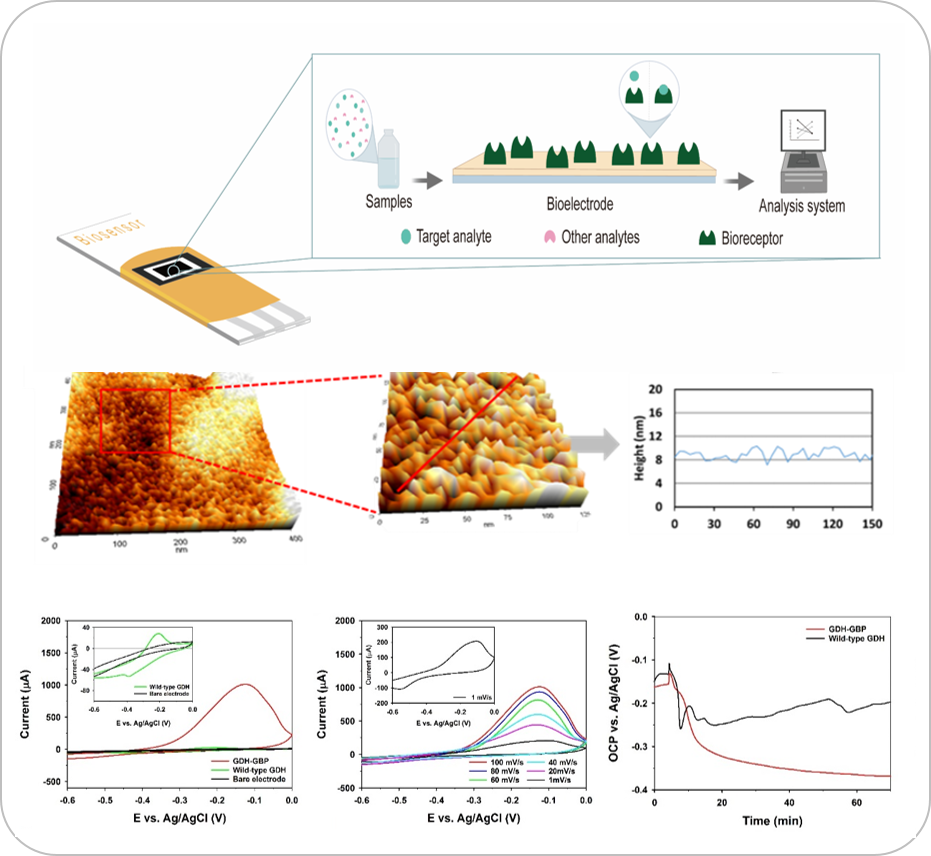
Enzymatic electrochemical biosensors enable in vivo quantification of specific biomarkers in real-time. However, the interface of such electroenzymatic biosensors is often far too large to monitor signalling molecules between two specific cells.
We are utilizing a combination of computational and experimental approaches for designing exogenous electron transport pathways to enable electroenzymatic biosensors that can operate in less than 100 nm of space. The development of high-precision strategies for biosensor design will enable continuous measurement of any number of signaling molecules between cells, for example specific neurotransmitters within a synapse or bioanalytes at a host-pathogen interface.
We are utilizing a combination of computational and experimental approaches for designing exogenous electron transport pathways to enable electroenzymatic biosensors that can operate in less than 100 nm of space. The development of high-precision strategies for biosensor design will enable continuous measurement of any number of signaling molecules between cells, for example specific neurotransmitters within a synapse or bioanalytes at a host-pathogen interface.
[Selected publications in this area]

Yoo-Seok Lee Ph.D.
Laboratory : Room 404, Building D, Tech University of Korea
Tel :+82-31-8041-0614
Email: y.lee@tukorea.ac.kr
